
Results & Side Effects
The floor is lava, but her skin is

Not an actual patient. Symptoms illustrated.

ZORYVE rhymes with “BELIEVE”
ZORYVE was tested as a once-daily, stand-alone treatment for 4 weeks in a clinical study involving 652 children ages 2–5 with mild to moderate eczema. 436 children used ZORYVE, and 215 children used an inactive cream (a cream not containing the active ingredient).
Clear or Almost Clear skin
25% of children using ZORYVE had Clear or Almost Clear skin in 4 weeks vs 11% of children using inactive cream
Additional Results*
These assessments were exploratory endpoints and reported by caregivers, which means these findings can help describe patterns but aren’t meant to draw definitive conclusions. Individual results may vary.
Itch Reduction
of 347 children using ZORYVE once daily reported itch reduction within 4 weeks vs 18% of 160 children using inactive cream.
Itch After Application
Some children experienced less itching within 24 hours after the first ZORYVE application.
*Itch levels were tracked and reported by caregivers of children daily. This technique may need more testing to confirm its accuracy in children under 12 years old. Results may vary.
Clearer skin you can see.
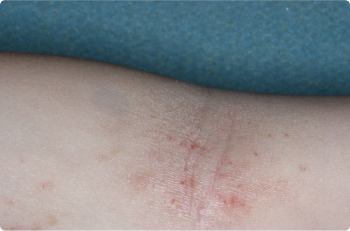
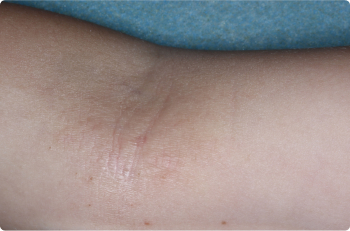
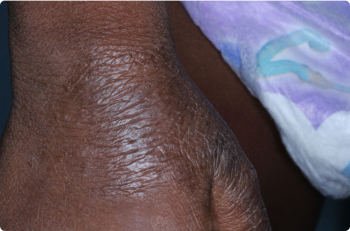
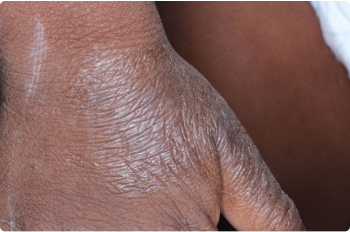
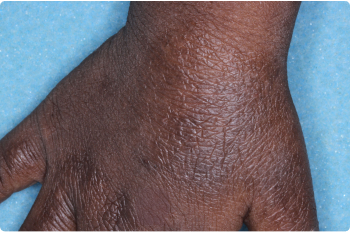
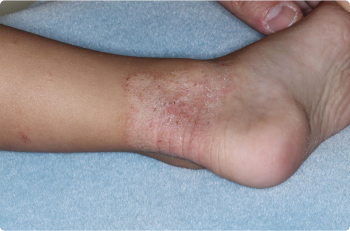
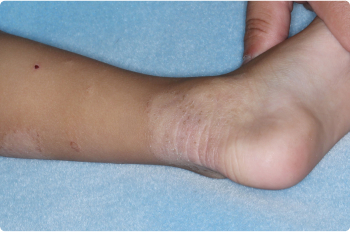
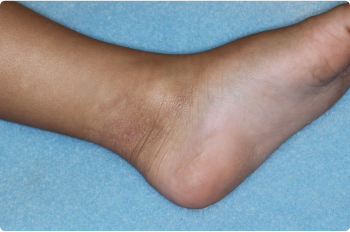
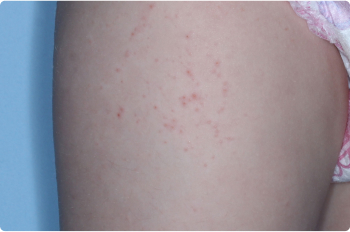
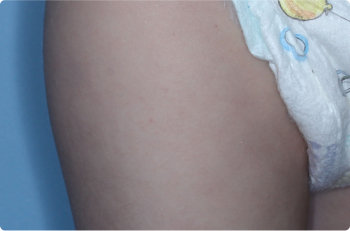
Actual clinical trial patients. Results may vary.
Ankle
3 YEAR OLD
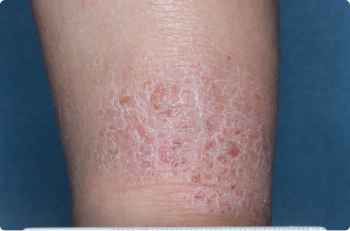
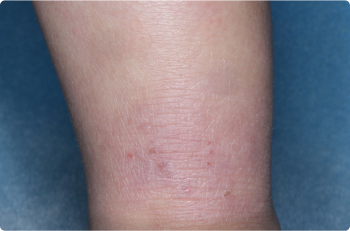
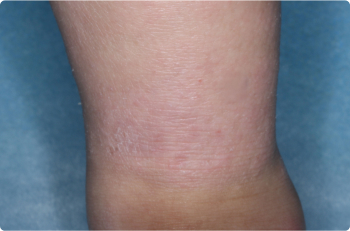
Inside Elbow
3 YEAR OLD
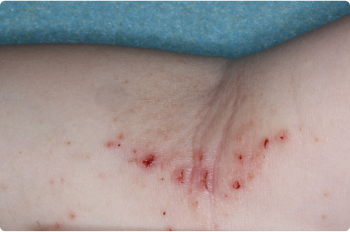
Hand
3 YEAR OLD
Ankle
3 YEAR OLD
Chest
2 YEAR OLD
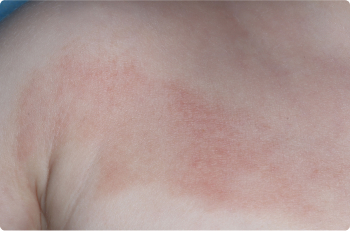
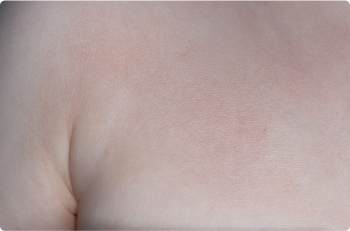
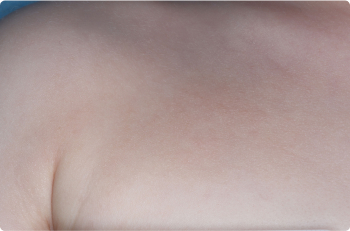
Foot
2 YEAR OLD
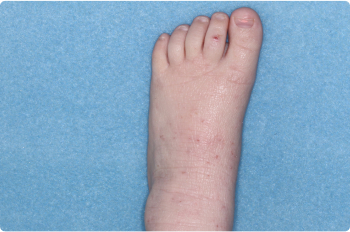
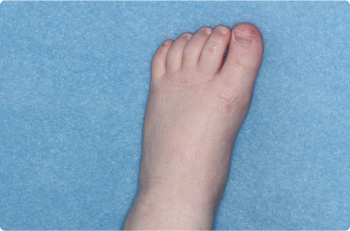
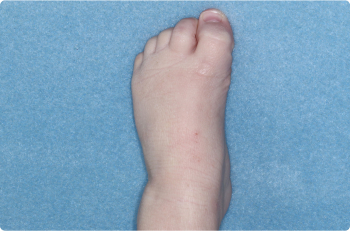
Thigh
2 YEAR OLD
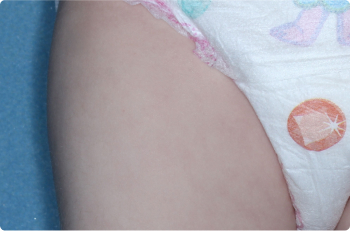
Back Of Knee
4 YEAR OLD
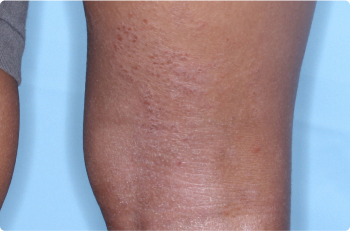
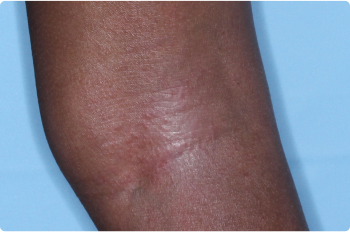
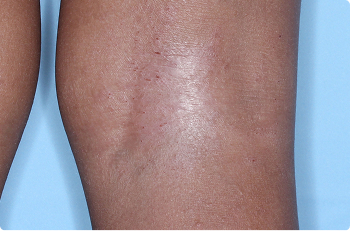
Wrist And Hand
4 YEAR OLD
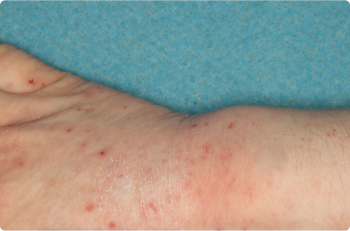
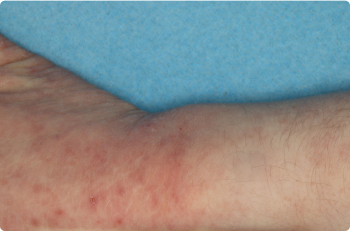
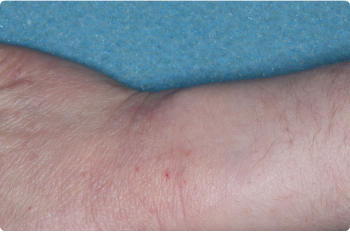
Before starting treatment with ZORYVE in the clinical trial, 52% of children previously used topical steroids that did not help or were not the right fit for them.
Does ZORYVE burn or sting?
Stinging or burning that caused discomfort was reported in less than 1% of children (age 2–5) within the first 10–15 minutes of applying ZORYVE vs 3.6% of children using inactive cream.
Most common side effects from clinical trials
These are not all the possible side effects of ZORYVE cream. Call your provider for medical advice about side effects.
ZORYVE is safe and can be used anywhere on the skin affected with eczema and for however long your child needs it.
Sign up for updates about ZORYVE
Get UpdatesTalk with your
dermatology provider
Not sure how to talk about your or your little one's atopic dermatitis? See our helpful Appointment Guide.
USE THE GUIDE